Supervision Administration
Zhu Xin, Gong Qianfei, Li Xiangyu
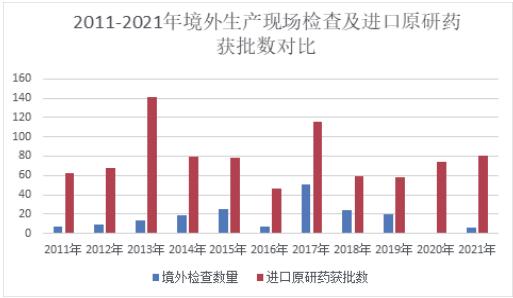
Objective: To provide countermeasures and suggestions for improving the standardization and systematization of GMP inspection management in China and ability improvement of inspection and risk elimination by a comparative study of GMP inspection standards and guidelines in China and the Pharmaceutical Inspection Co-operation Scheme (PIC/S). Methods: Through literature research, the implementation of risk-based GMP inspection plans for drugs by PIC/S, European and American drug regulatory authorities was analyzed, and the path of implementing risk-based GMP inspection plans in China was explored. Results and Conclusion: The concept of formulating GMP inspection plans based on risk has been widely implemented in PIC/S, European and American drug regulatory inspection systems, and has formed a relatively mature model applicable locally, giving full play to the eff ectiveness of intensive inspection resources and eff ective investigation of hidden dangers. Based on the relevant requirements for inspection procedures in China's drug regulatory system, this paper analyzes the implementation status and challenges of risk-based GMP inspection plans in China, and puts forward countermeasures and suggestions from the aspects of formulating risk-based inspection plans, detailed inspection procedures, and ensuring inspection resources, so as to unify and standardize the operation mechanism and standards of drug inspection by drug regulatory authorities and improve the effi ciency of drug inspection.